nm4bl2104 – Bioelectronic capture and release of cancer cells
Poster-Final
Bioelectronic capture and release of cancer cells
Janire Saez1,2,3*, Maite Garcia-Hernando2,4, Achilleas Savva1, Lourdes Basabe-Desmonts2,3, Fernando Benito-Lopez4*and Róisín M. Owens1
1 Department of Chemical Engineering and Biotechnology, University of Cambridge, U.K.
2 Microfluidics Cluster UPV/EHU, BIOMICs microfluidics Group, Lascaray Research Center, University of the Basque Country UPV/EHU, Spain
3 Basque Foundation of Science, IKERBASQUE, Spain
4 Microfluidics Cluster UPV/EHU, Analytical Microsystems & Materials for Lab-on-a-Chip (AMMa-LOAC) Group, Analytical Chemistry Department, University of the Basque Country UPV/EHU, Spain.
ABSTRACT
Here, we present a novel thermo-responsive material, poly(N-isopropylacrylamide) combined with an electroactive material, poly(3,4-ethylene-dioxythiopene):poly(styrene sulfonate) for the generation of label-free and non-invasive detection of the capture and release of cells on bioelectronic devices. The copolymer simultaneously monitors electrically the capture and release of cells, and undergoes a conformational change, releasing the capture cells, in response to temperature. This platform could be applied at the point-of-care to other cells obtained in clinic.
KEYWORDS: T-responsive, Smart Functional Materials, Electrochemical Impedance Spectroscopy, Cancer.
INTRODUCTION
The development of miniaturized devices capable of non-invasive collection of circulating tumor cells has found important applications in cancer research.1 In this application, cells usually need to be analyzed after their collection, so guaranteeing their viability during the process is essential.
Smart functional materials such as poly(N-isopropylacrylamide) (pNIPAAm) can undergo structural changes due to their inherent lower critical solution temperature (LCST) phase transition in water at 32 ºC. Below the LCST, the polymer swells due to hydrogen bond interactions between chains. Above the LCST, the disruption of the hydrogen bonding interactions causes the material to shrink.2 pNIPAAm has proven to be compatible and viable to modulate cellular adhesion/detachment.3 On its side, poly(3,4-ethylenedioxythiopene):poly(styrene sulfonate) (PEDOT:PSS) is a widely used conducting polymer in the bioelectronics field, due to its mixed ionic and electronic conduction properties. PEDOT:PSS-based electrodes have previously been used for monitoring cell attachment and proliferation by electrochemical impedance spectroscopy (EIS).4
This work presents, a novel electroactive functional copolymer (PEDOT:PSS/pNIPAAm),which combines the conducting properties of the PEDOT:PSS and the thermo-actuation capabilities of pNIPAAm. This material enables the label-free capture and release of cells with simultaneous EIS monitoring of the process (Figure 1A).
EXPERIMENTAL
Gold-electrodes were fabricated by following this protocol (Figure 1B).5 PEDOT:PSS/pNIPAAm was prepared by mixing 452 mg pNIPAAm, 30 mg of N,N’-methylenebis(acrylamide) (mBAAm), 2,2-dimethoxy-2-phenylacetophenone (DMPA) as photoinitiator and 2 mL of PEDOT:PSS for 2h. 62 μL of glycidoxy propyltrimethoxysilane (GOPS, PEDOT:PSS crosslinker) was added and left to stir for 30 min (Figure 1C), and then filtered. PEDOT/pNIPAAm copolymer was spin coated at 3000 rpm for 30 s and soft baked, UV treated and hard baked for 1 min, 30 s and 2 min, respectively. For the capture of cells sw480 adenocarcinoma cancer cells were captured by embedding fibronectin protein into the copolymer for 2 h, following with three cell media washes. Then, 650.000 cells in 100 μL media were added to the PEDOT:PSS/pNIPAAm electrodes and left to attach for 3 h at rt. Cell release was triggered by applying 37 °C for 20 min on a hot plate, cells were collected and electrodes were washed with media. Bright-field pictures and EIS measurements were taken after each step.
RESULTS AND DISCUSSION
The impedance data obtained during cell capture and release experiments on PEDOT:PSS/pNIPAAm electrodes displayed an increased impedance at the mid-high frequencies (> 10 Hz) for the samples with cells at 23 ºC, with a subsequent recovery of impedance values when temperature-triggered cell release happened (Figure 1D). The Rcells values were calculated for the different stages of the process with PEDOT:PSS/pNIPAAm electrodes (Figure
2E, left) by fitting the experimental data to a R(RC)(RC) circuit; a R for the electrolyte and two parallel RC circuits, one for the electrode and another for the cell layer on top of the polymer film (Figure 2E, right). Data showed a Rcells reduced value of PEDOT:PSS/pNIPAAm after heating the electrodes (actuation), which corresponds to a 67 % drop value compared to the Rcells calculated after cell capture, suggesting that cells were released from the electrode. These results were also supported by optical microscopy (Figure 2F), where cells detached from the PEDOT:PSS/pNIPAAm surface, and returned to the media (more than 90 % of cells detached from all the electrodes). The non-invasiveness of the technique was evaluated by adding trypan blue to the released cells, which confirmed a cell viability of 94 % ± 1 after the cell capture and release process.
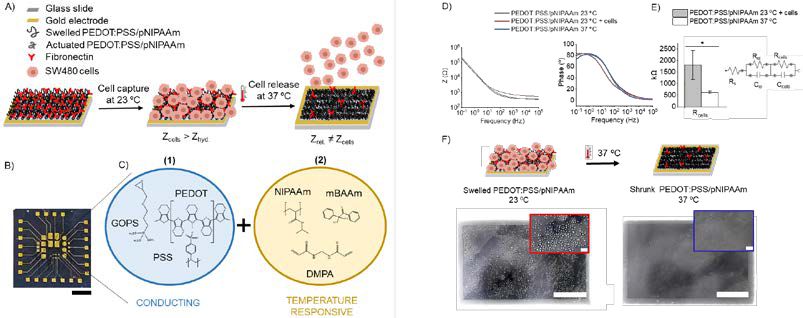
Figure 1: A) Schematics of the cell capture and release mechanism based on the hybrid functional PEDOT:PSS/pNIPAAm copolymer and electrical monitoring of the process. B) Photograph showing the photolithographically patterned device consisting of 24 gold-electrode arrays on glass. Scale bar:5 mm. C) Chemical structure of the components of (1) the conducting polymer: PEDOT and PSS with GOPS and (2) the thermo-responsive polymer: NIPAAm, mBAAm, and DMPA. PEDOT:PSS/pNIPAAm D) Impedance vs frequency and phase vs frequency plots, E) Rcells plotted with cells at 23 ºC and at 37 ºC after heating (n = 4). * significantly different (0.05, one-way ANOVA, p = 0.009). Inside: R(RC)(RC) electric circuit fitting for this system. F) Scheme of the actuation process and bright-field images of the captured cells (left) and release (right) (4x, scale bar 400 μm) with a zoomed fraction of 400 x 200 μm area (scale bar 40 μm).
CONCLUSION
In conclusion, the novel PEDOT:PSS/pNIPAAm is functional for temperature triggered release of captured cells, as well as for the simultaneous monitoring of the process by EIS. This opens the possibility of using organic bioelectronics as label-free devices, leading to an easy tracking of the process, minimizing user intervention, towards development of more complex architectures for simultaneously sensing and actuating different targets.
ACKNOWLEDGEMENTS
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 842356, Gobierno de España, Ministerio de Economía y Competitividad, with Grant No. BIO2016-80417-P (AEI/FEDER, UE) and Gobierno Vasco research groups (IT1271-19).
REFERENCES
[1] H. Ruan et al. “A Supersensitive CTC Analysis System Based on Triangular Silver Nanoprisms and SPION with Function of Capture, Enrichment, Detection, and Release” ACS Biomater. Sci. Eng. 4, 1073-1082, 2018.
[2] S. Gallagher. et al. “Ionic liquid modulation of swelling and LCST behavior of N-isopropylacrylamide polymer gels“ Phys. Chem. Chem. Phys. 16, 3610-3616, 2014.
[3] A. Choi et al. “Bulk poly(N-isopropylacrylamide) (PNIPAAm) thermoresponsive cell culture platform: toward a new horizon in cell sheet engineering“ Biomater. Sci. 7, 2277-2287, 2019.
[4] I. Iandolo et al. “Biomimetic and electroactive 3D scaffolds for human neural crest-derived stem cell expansion and osteogenic differentiation” MRS Commun. 10, 179-187, 2020.
[5] P. Cavassin et al., “Organic Transistors Incorporating Lipid Monolayers for Drug Interaction Studies“ Adv. Mater. Technol., 5, 1900680, 2019.