nm4bl2114 – ALGINATE BEAD BIOSENSORS FOR THE DETERMINATION OF LACTATE LEVELS USING IMAGE ANALYSIS
NM4BL-Poster-Sandra-Garcia-Rey
ALGINATE BEAD BIOSENSORS FOR THE DETERMINATION OF LACTATE LEVELS USING IMAGE ANALYSIS
Sandra Garcia-Rey1,2, Edilberto Ojeda1,2, Udara Bimendra Gunatilakea,b, Lourdes Basabe-Desmonts2,3,4,5,*, and Fernando Benito-Lopez1,2,3,*
1Microfluidics Cluster UPV/EHU, Analytical Microsystems & Materials for Lab-on-a-Chip (AMMa-LOAC) Group, Analytical Chemistry Department, University of the Basque Country UPV/EHU, Spain
2Microfluidics Cluster UPV/EHU, BIOMICs microfluidics Group, Lascaray Research Center, University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain
3Bioaraba Health Research Institute, Microfluidics Cluster UPV/EHU, Vitoria-Gasteiz, Spain
4BCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, Leioa, Spain
5Basque Foundation of Science, IKERBASQUE, María Díaz de Haro, 3, 48013 Bilbao, Spain
ABSTRACT
The potential to determine lactate concentrations in physiological fluids, at the point of need with minimal invasiveness, is very valuable in sport science and medicine. In this work, the synthesis and performance of alginate bead biosensors for lactate sensing was investigated. Lactate in artificial sweat was detected with an R² = 0.9907 in a linear range from 10 mM to 100 mM, with a limit of detection of 6.4 mM and a limit of quantification of 21.2 mM. This novel sensing configuration gives a fast and reliable method for lactate sensing, which could be integrated into more complex analytical systems.
KEYWORDS: lactate, sweat, alginate bead, biosensor, colorimetric analysis
INTRODUCTION
Sweat is an aqueous solution that provides a high amount of physiological information1, which together with its accessibility in a non-invasive way, highlights its potential as an emerging alternative to standard blood analysis. In particular, lactate, which is a product of the anaerobic metabolism that takes place during intense exercise, can be used as a biomarker to keep track of the performance of athletes. This states the importance of finding new methods for the determination of lactate levels in sweat, such as alginate, which is a natural and biocompatible anionic polymer that can be crosslinked creating a 3D network. In this work, we present the fabrication and characterization of alginate beads and the integration of the enzymatic assay for the determination of lactate levels in artificial sweat.
THEORY
The reaction taking place inside the alginate bead is shown in Figure 1. After being added, lactate solution covered the alginate bead while the enzymatic components of the mix remained inside the bead. Then, diffusion started in both directions, this is, lactate entered the bead while LOD, HRP and TMB slowly diffused outside.
EXPERIMENTAL
The reaction mix for lactate sensing consists of 10 μL of lactate oxidase (LOD, 1 KU) 0.40 mg mL-1, 10 μL of horseradish peroxidase (HRP, 200 U) 0.05 mg mL-1, 5 μL of 24 mg of tetramethyl benzidine dissolved in 2.25 mL of dimethyl sulfoxide (TMB:DMSO) and alginate 1.5 %. For the fabrication of the sensor, 25 μL of the reaction mix were dipped in CaCl2 400 mM and the beads were washed with distilled water. Solutions of lactate 10, 20, 40, 60, 80 and 100 mM were spiked in artificial sweat (NaCl, urea and sodium L-lactate 60 mM in distilled water) and the pH was adjusted to 6.5. 50 μL of these solutions were added to the beads and images were taken during 1 h. Using the image color analysis software ImageJ, the black and white value (B/W value) of each bead was measured.
RESULTS AND DISCUSSION
The fabricated alginate bead sensors (Figure 2) were clear and uniform, and presented a well-defined spherical shape with a diameter of 2.3 ± 0.2 mm (n = 20). Alginate concentration was optimized to 1.5 % (Figure 3). As can be observed in Figure 4A, at higher lactate concentrations, the enzymatic reaction was shifted towards the complete
oxidation of the TMB, this is, the formation of the yellow product thus, higher B/W values. The calibration curve, Figure 4B, was built at min 13 and the lactate concentrations were determined in a linear calibration range of 10 to 100 mM, which is compatible with the sweat lactate values obtained in literature2. A limit of detection of 6.4 mM and a limit of quantification of 21.2 mM were obtained. The alginate sensor was verified with two solutions of lactate 20 mM and 45 mM in artificial sweat, obtaining values of 18 ± 2 and 50 ± 7 mM, respectively (error bars correspond to mean values ± SD, n = 3).
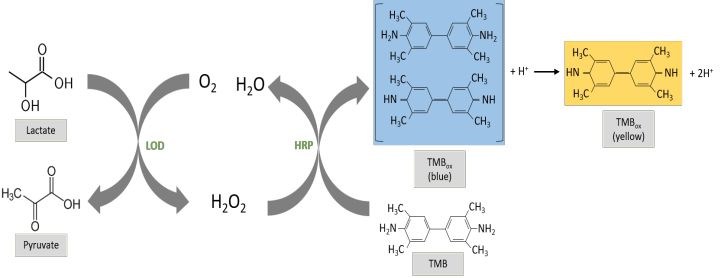
Figure 1:
Enzymatic cascade for lactate detection. The LOD (lactate oxidizes the lactate to pyruvate yielding H 2 O 2 . Using it as the electron donor , the HRP (horseradish peroxidase) performs the oxidation of TMB, yielding a blue color product, which can be further oxidized in a two electron oxidation as more H 2 O 2 is generated, obtaining an orange/yellow colored product.
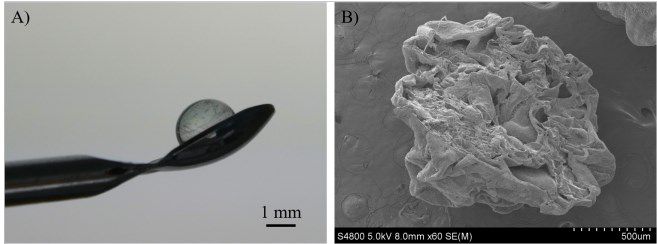
Figure 2:
Alginate bead sensor. A) Image of the bead and B) a SEM image of the sensor.
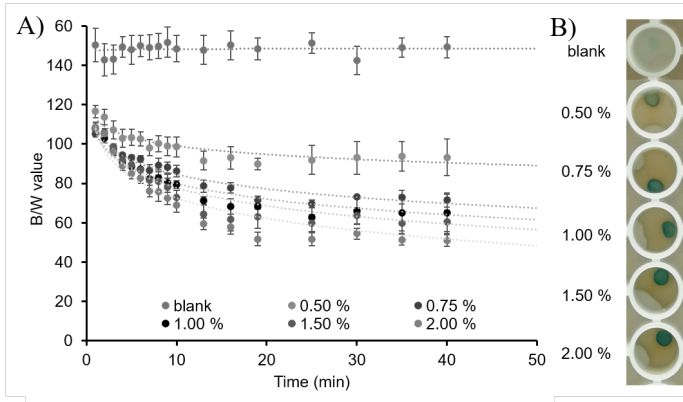
Figure 3:
Optimization of the alginate concentration for the fabrication of alginate beads. A) B/W values of the beads with alginate 0.5, 0.75, 1, 1.5 and 2 %, after adding lactate 60 mM in artificial sweat. B) Pictures of beads with alginate concentrations of 0.5, 0.75, 1, 1.5 and 2 % taken at 13 min time, when the reaction reached plateau.
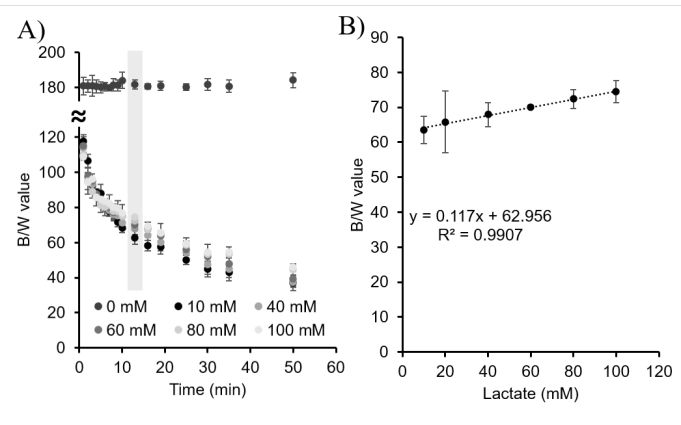
Figure 4:
A) B/W values of alginate beads tested with artificial sweat containing 0, 10, 20, 40, 60, 80 and 100 mM concentration of lactate. The shadowed values indicate the time when the plateau is reached, 13 min. B) External calibration line at 13 min for the determination of lactate in the range of 10 mM to 100 mM in artificial sweat.
CONCLUSION
In this work we have demonstrated a new alginate bead sensor approach for lactate sensing in artificial sweat. With lactate being a biomarker of the anaerobic metabolism, the research developed here opens a door to a new way of lactate determination using alginate with applications in sports science and medicine, as well as new avenues in sensor devices.
ACKNOWLEDGEMENTS
The authors acknowledge support from Gobierno de España, MINECO (Grant No. BIO2016-80417-P) (AEI/FEDER, UE), the Basque Government (Grant IT1271-19), the EU DNASurf (H2020-MSCA-RISE-778001) and the EU Horizon 2020 research and innova-tion program under grant agreement No. 766007.
REFERENCES
[1] Bariya, M.; Nyein, H. Y. Y.; Javey, A. Wearable Sweat Sensors. Nature Electronics. 2018, pp 160–171.
[2] Patterson, M. J.; Galloway, S. D. R.; Nimmo, M. A. Variations in Regional Sweat Composition in Nor-mal Human Males. Experimental Physiology 2000, 85 (6), 869–875.