nm4bl2113 – IONOGEL BASED MATERIAL FOR THE COLORIMETRIC DETECTION OF Δ9-TETRAHYDROCANNABINOL
RaquelCatalan
IONOGEL BASED MATERIAL FOR THE COLORIMETRIC DETECTION OF Δ9-TETRAHYDROCANNABINOL
Raquel Catalan-Carrio1, Guillermo Moreno-Sanz2, Lourdes Basabe-Desmonts1,3,4,5,*, Fernando Benito-Lopez1,3,4,*
1Microfluidics Cluster UPV/EHU, University of the Basque Country UPV/EHU, Spain
2ABAGUNE RESEARCH, Vitoria-Gasteiz, Spain
3Bioaraba Health Research Institute, Vitoria-Gasteiz, Spain
4BCMaterials, Basque Centre for Materials, Micro and Nanodevices, UPV/EHU Science Park, Leioa, Spain
5Basque Foundation for Science, IKERBASQUE, Spain
{raquel.catalan, lourdes.basabe, fernando.benito}@ehu.eus
ABSTRACT
Cannabis sativa is the most widely abused illegal drug in the world. Additionally, there is an increasing interest in the production of non-psychoactive industrial hemp for several applications, including the production of cannabidiol (CBD). This makes the easy and fast in situ detection of Δ9-tetrahydrocannabinol or THC (psychoactive substance of cannabis) a pressing need. It is here where detection mechanisms based on novel methodologies e.g. microfluidics devices and new materials able to produce portable devices, and to perform fast analysis with a reduction of reagents consumption, can be of great importance. At the same time, portability can be improved by using solid matrixes, where the detection mechanism could take place, such as an ionogel. In this work, we report for the first time the use of an ionogel matrix for the colorimetric detection of THC through the Fast Blue B Salt method. The integration of the Fast Blue B Salt into the ionogel matrix was studied as a first step in the development of a fully integrated sensor, for the identification of THC from hemp biomass and extracts.
KEYWORDS: materials, Fast Blue B Salt, cannabinoid detection, ionogel, microfluidics
INTRODUCTION
The consumption of illicit drugs is a safety and health concern worldwide, among all the drugs of abuse, cannabis is the most consumed [1]. The need for a fast detection and control of these substances implies the development of novel alternatives to traditional methods, which should be costs effective both economically and environmentally. At this point, the integration of a colorimetric method of detection and analysis into a miniaturised device format would be of great interest. For instance, one benefit of using microfluidic devices is the possibility of an easy incorporation of semi-solid matrixes that can act as sensing materials. In particular, ionogels based on N-isopropyl acrylamide (PNIPAM) have been employed for pH sensing [2] and nitrate/nitrite detection [3], among others. Ionogels are hybrid materials that incorporate a polymer into an ionic liquid (IL), maintaining the properties of the IL like its thermal stability and solubility.
Miniaturised devices, ready to be used in none controlled environments out of the laboratory, require easy to use detection methods. Therefore, colorimetric analysis is gaining attention [4]. When using colorimetric reactions, photos or videos image analysis have being developed, including smartphone detection [5], for in environmental and health applications [6]. For our application, an advantage of the detection by a smartphone, when analysing illicit drugs, is the possibility to easily communicate the results to the authorities.
Hence, in this contribution, we present the first step toward the development of a microfluidic device for THC detection, focusing on the integration of the detection assay on an ionogel matrix, for colorimetric analysis.
EXPERIMENTAL
Polymethyl methacrylate (PMMA) devices were designed and fabricated using a CO2 laser ablation system. The design had two reservoirs, one to monitor the colour change of the ionoge without sample (blank) and another where the sample was added (sample). Each reservoir was designed for a final volume of 12 μL of ionogel and sample with containing walls to prevent the spreading of the sample from the ionogel to the PMMA surface due to the hydrophobicity of the ionogel, Fig. 1.
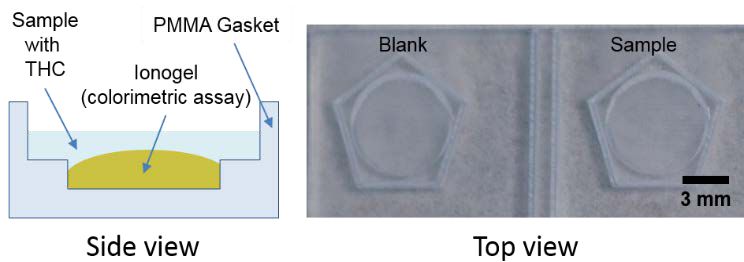
Fig. 1.(Left) Scheme of the gasket for the investigation of the ionogel performace, side view. (Right) Image of the gasket before use. The circular part corresponds to the reservoir for the ionogel and the reaction, while the pentagon is the containing wall to prevent the spreading of the sample.
Ionogels were synthetised following a previously reported work [3], using N-isopropylacrylamide, N,N’-methylene-bis(acrylamide) monomers and 2,2-dimethoxy-2-phenylacetophenone as photoinitiator, dissolved in different ILs, with different hydrophobicity to study the affinity of THC to matrixes with different hydrophobicity (Table 1). Then, the incorporation of the solid reagent Fast Blue B Salt (FBBS) into the monomeric mixture before the polymerization, was also investigated. For the polymerization of the ionogel matrix, 12 μL of each ionogel prepolymer solution was pipetted into the two reservoirs of the gaskets and polymerised using a UV-Vis lamp. For the IO-I to IO-III ionogels 15 min was enough time to obtain the ionogel, while for IO-IV and IO-V ionogels 50 and 90 min were required, respectively.
The colorimetric assay was optimized to fulfil the low solubility requirement of FBBS in water, obtaining that the best volume ratio was found to be 7:1:1 (FBBS:NaOH:ethanol). THC was provided as a hemp extract solution in ethanol containing 12.5, 1.25 and 0.125 mg mL-1 of THC.
For the colorimetric reaction, two different procedures were used depending of the type of ionogel. For IO-I to IO-III, since the FBBS was not incorporated into the ionogel mixture, 12 μL were pipetted into both reservoirs and left to dry for 20 min. For IO-IV and IO-V, FBBS was already polymerised within the ionogel thus, THC was directly pipetted on the ionogel, the colorimetric reaction was left for 20 min to take place. In addition, in order to improve the possible colorimetric response of the reaction, FBBS was pipetted twice.
Images of the devices were taken with a Canon 1600D camera and the Red Green Blue (RGB) values were analysed with Adobe Photoshop. Since the aim of this work was to obtain a qualitative detection of a colour change under the presence of THC in the ionogel matrix and not a quantification of the concentration of THC, a simple image analysis method, using the RGB model was enough to monitoring the colour.
RESULTS AND DISCUSSION
FBBS was not soluble in 1-ethyl-3-methylimidazolium ethyl sulfate, while for the other two ionic liquids, IO-IV and IO-V, a dark and a light yellow liquid, were obtained respectively. The integration of the FBBS into the ionogel matrix is of great interest for the development of the sensor, since FBBS is considered as a carcinogenic substance. The integration into a solid-like material would reduce its manipulation and reduce waste.
All the ionogels tests gave a colour change was observed by bare eye. The lower concentration distinguished by bare eye was obtained using the ionogel IO-I (0.125 mg mL-1), while all the other four ionogels, required higher concentrations of THC to have a distinguishable colour from the blank (1.25 mg mL-1). The ionogel IO-IV presented some drawbacks, since the colour obtained was not homogeneously distributed within the ionogel matrix, and the reaction was obtained in red spots, probably due to the low solubility of FBBS in the ionogel mixture. On the other hand, the ionogel IO-V did not present any solubility problems for the FBBS into the ionogel mixture, but the reaction was found to be very slow, up to 5 h. The performance of the ionogel IO-V could be improved by increasing the amount of FBBS into the ionogel prepolymer solution.
In order to be able to quantify the performance of the gasket, image analysis of the pictures taken after 20 min was carried out. The decrease of the values in the RGB colour model, between the blank reservoir (left) and the THC reaction reservoir (right), were calculated. The best results to observe the colour change were obtained for the G value, shown in Fig. 2.
Table 1 Different ionic liquids used.
aFBBS incorporated or not into the ionogel prepolymer solutio
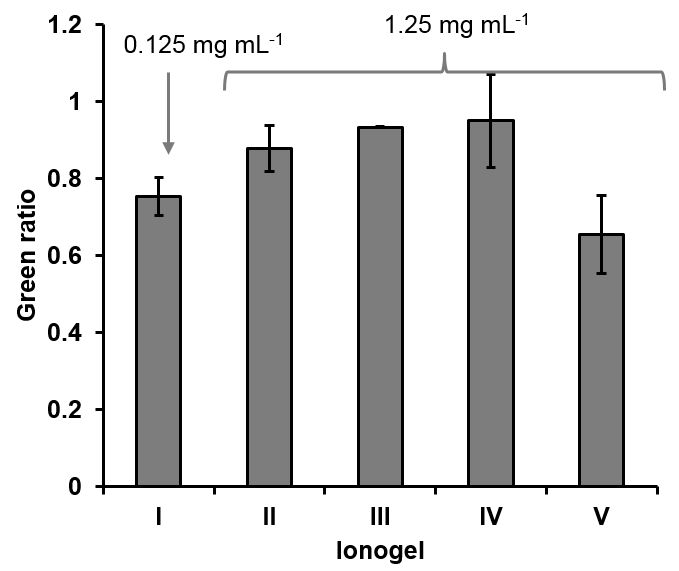
Fig. 2.Values obtained for the ratio between the Green value for the FBBS and the Green value after the reaction with THC takes place in the different ionogel matrixes. I to V correspond to the name of the ionogels mentioned before, and the corresponding concentrations of THC at whitch those colours were obtained. Error (n = 3, devices)
CONCLUSIONS
In this work, the use of ionogels as semi-solid matrixes for the colorimetric detection, with the bare eye, of THC from hemp tracts was investigated. Among all the ionogel used, the one based on 1-ethyl-3-methylimidazolium dicyanamide was the one detecting the lower concentration of THC, 0.125 mg mL-1 (IO-I). Nevertheless, the same ionogel but incorporating the FBBS in one single step presented good solubility of the reagent but the reaction time was found to be high, 5 h. These two ionogel mixtures seem promising for the detection of THC from hemp extracts.
ACKNOWLEDGEMENTS
The authors would like to acknowledge funding support from Gobierno de España, Ministerio de Economía y Competitividad, with Grant No. BIO2016-80417-P (AEI/FEDER, UE), the Gobierno Vasco Dpto. Educación for the consolidation of the research groups (IT1271-19). This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 778001. This work was also partially funded by ABAGUNE RESEARCH.
REFERENCES
[1] B. J. Mano-Sousa et al. “Color determination method and evaluation of methods for the detection of cannabinoids by thin-layer chromatography (TLC).” Journal of Forensic Science 66.3 (2020): 854-865.
[2] V. F. Curto et al., “Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic plat- form incorporating ionic liquids”, Sens. Actuators B, 171-172, 1327-1334, 2012.
[3] J. Saez et al., “Ionogel-based Nitrite and Nitrate Sensor for Water Control at the Point-of-Need” Proc. Eng, 168, 518-521, 2016.
[4] G. G. Morbioli, T. Mazzu-Nascimento, A. M. Stockton and E. Carrhilho, “Technical aspects and challenges of colorimetric detection with microfluidic paper-based analytical devices (μPADs) – A review.” Analytica Chimica Acta 970 (2017): 1-22
[5] R. Jain, A. Thakur, P. Kaur, K. Kim, and P. Devi,” Advances in imaging-assisted sensing techniques for heavy metals in water: Trends, challenges, and opportunities.” Trends in analytical chemistry 123 (2020): 115758
[6] N. Yamaguchi and Y. Fujii, “Rapid On-Site Monitoring of Bacteria in Freshwater Environments Using a Portable Microfluidic Counting System.” Biological & pharmaceutical bulletin 43 (2020): 87-92